GIBBS DONNAN EFFECT
SUMMARY
1. Donnan & Gibbs showed that in the presence of a non-diffusable ion, diffusable ions distribute themselves across the membrane so that electro-chemical equilibrium is achieved:
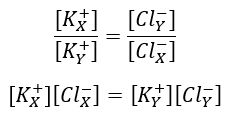
2. Because of greater intracellular charged protein concentration, the inside of the cell is hypertonic. The cell would eventually swell and rupture if not for Na+/K+-ATPease pumping ions out of the cell.
3. Because at chemical equilibrium the distribution of permeant ions across the membrane is asymmetric, an electrical gradient exists across the membrane as described by the Nernst equation.
4. Because there are more proteins in plasma than interstitium, there is a Donnan effect on ion movement across capillary walls.
Reference(s)
Barrett, K.E., Barman, S.M., Brooks, H.L., X, J. and Ganong, W.F. (2019). Ganong’s review of medical physiology. 26th ed. New York: Mcgraw-Hill Education