OSTEOPOROSIS-MANAGEMENT (TERIPARATIDE)
SUMMARY
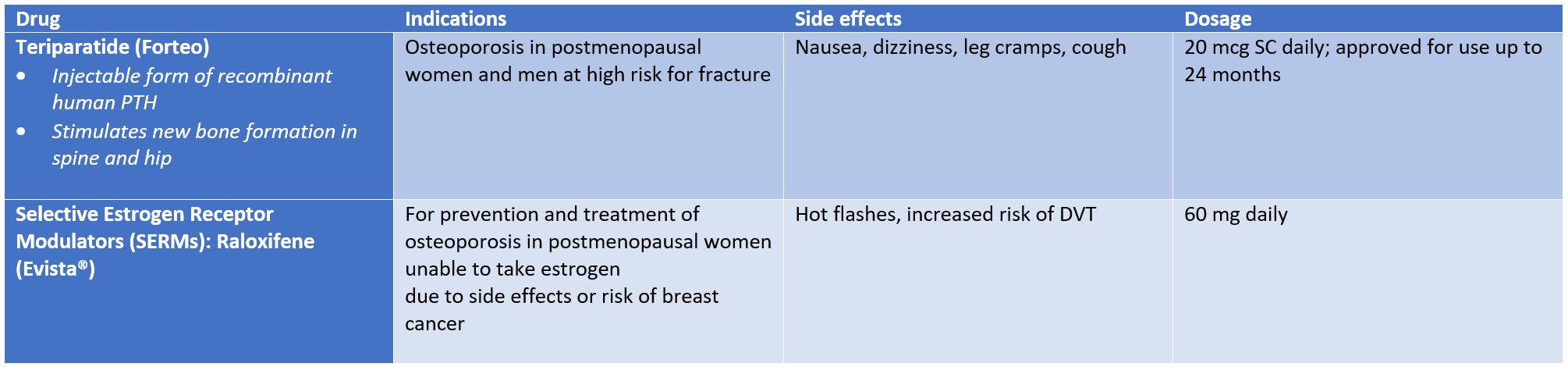
1. Injectable form of recombinant human PTH, stimulates new bone formation in the spine and hip (osteoblastic activity).
2. Indications: OP in post-menopausal women & men at high risk of fractures, when other agents have failed, primary OP, steroid-induced OP.
3. Side effects: osteosarcoma, nausea, dizziness, leg cramps, cough.
4. Dosage: 20mcg SC daily; approved for use up to 24 months.
Reference(s)
Wilkinson, I., Furmedge, D. and Sinharay, R. (2017). Oxford handbook of clinical medicine. Oxford: Oxford University Press. Get it on Amazon.
Feather, A., Randall, D. and Waterhouse, M. (2020). Kumar And Clark’s Clinical Medicine. 10th ed. S.L.: Elsevier Health Sciences. Get it on Amazon.
Hannaman, R. A., Bullock, L., Hatchell, C. A., & Yoffe, M. (2016). Internal medicine review core curriculum, 2017-2018. CO Springs, CO: MedStudy.
Therapeutic Guidelines. Melbourne: Therapeutic Guidelines Limited. https://www.tg.org.au [Accessed 2021].