Difference between revisions of "PH & BUFFERING-HENDERSON HASSELBACH EQUATION"
From NeuroRehab.wiki
(Imported from text file) |
(Imported from text file) |
||
| Line 10: | Line 10: | ||
==Reference(s)== | ==Reference(s)== | ||
Barrett, K.E., Barman, S.M | Barrett, K.E., Barman, S.M., Brooks, H.L., X, J. and Ganong, W.F. (2019). Ganong’s review of medical physiology. 26th ed. New York: Mcgraw-Hill Education | ||
[[Category:Ph & Buffering]] | [[Category:Ph & Buffering]] | ||
[[Category:Physiology]] | [[Category:Physiology]] | ||
Latest revision as of 02:30, 21 March 2023
SUMMARY
1. This equation, which can be applied to any buffer system, defines the relationship between dissociated and undissociated acids & bases.
2. It is mainly used to describe the equilibrium of the bicarbonate system.
3. Henderson-Hasselbach equation:
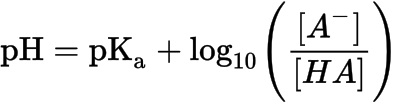
Reference(s)
Barrett, K.E., Barman, S.M., Brooks, H.L., X, J. and Ganong, W.F. (2019). Ganong’s review of medical physiology. 26th ed. New York: Mcgraw-Hill Education