Difference between revisions of "PH & BUFFERING-HENDERSON HASSELBACH EQUATION"
From NeuroRehab.wiki
(Imported from text file) |
(Imported from text file) |
||
| Line 14: | Line 14: | ||
<br/>West, J.B. and Luks, A.M. (2021). West’s Pulmonary Pathophysiology. Lippincott Williams & Wilkins. | <br/>West, J.B. and Luks, A.M. (2021). West’s Pulmonary Pathophysiology. Lippincott Williams & Wilkins. | ||
[[Category: | [[Category:Ph & Buffering]] | ||
[[Category:Physiology]] | [[Category:Physiology]] | ||
Revision as of 11:40, 2 January 2023
SUMMARY
1. This equation, which can be applied to any buffer system, defines the relationship between dissociated and undissociated acids & bases.
2. It is mainly used to describe the equilibrium of the bicarbonate system.
3. Henderson-Hasselbach equation:
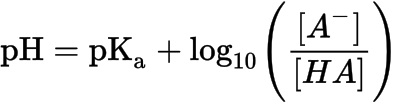
Reference(s)
Barrett, K.E., Barman, S.M., Boitano, S., Brooks, H.L., Weitz, M., Brian Patrick Kearns, Ganong, W.F. and Mcgraw-Hill Education (Firm (2016). Ganong’s review of medical physiology. 25th ed. New York: Mcgraw Hill Education.
Hall, J.E. and Hall, M.E. (2020). Guyton And Hall Textbook Of Medical Physiology. 14th ed. S.L.: Elsevier - Health Science.
West, J.B. and Luks, A.M. (2021). West’s Pulmonary Pathophysiology. Lippincott Williams & Wilkins.